New FDA Supplement Facts Box Labeling Changes: Thorne’s Ahead of the Game

Beginning in January 2020, supplement companies will be required to comply with several new FDA labeling regulations. At Thorne, we decided to get ahead of the game. So, along with our new rebranded labels, you will also see changes to the way many of the ingredients are listed on our product labels. This blog will help you navigate these changes, which admittedly can be a bit confusing.
Looks different but it’s the same formula
In most cases, the actual product formula will not change, but with the required changes they could look like they have. It might look like there is more or less of a certain ingredient, when that isn’t the case at all. Or you might not know how to interpret the amount of a particular ingredient because it’s listed in different units than you are accustomed to. For example, if you’re like me, you’ve always referred to vitamin A in International Units (IUs), so you’ll have no idea what that translates to in micrograms (mcgs). Hopefully this blog will help.
Vitamin changes: vitamin A, beta-carotene, vitamin D, vitamin E, folate, and choline
Let's look at some of the changes – starting with the way some of the vitamins will appear on the label. In the case of our newly branded labels, most of these changes have already taken place.
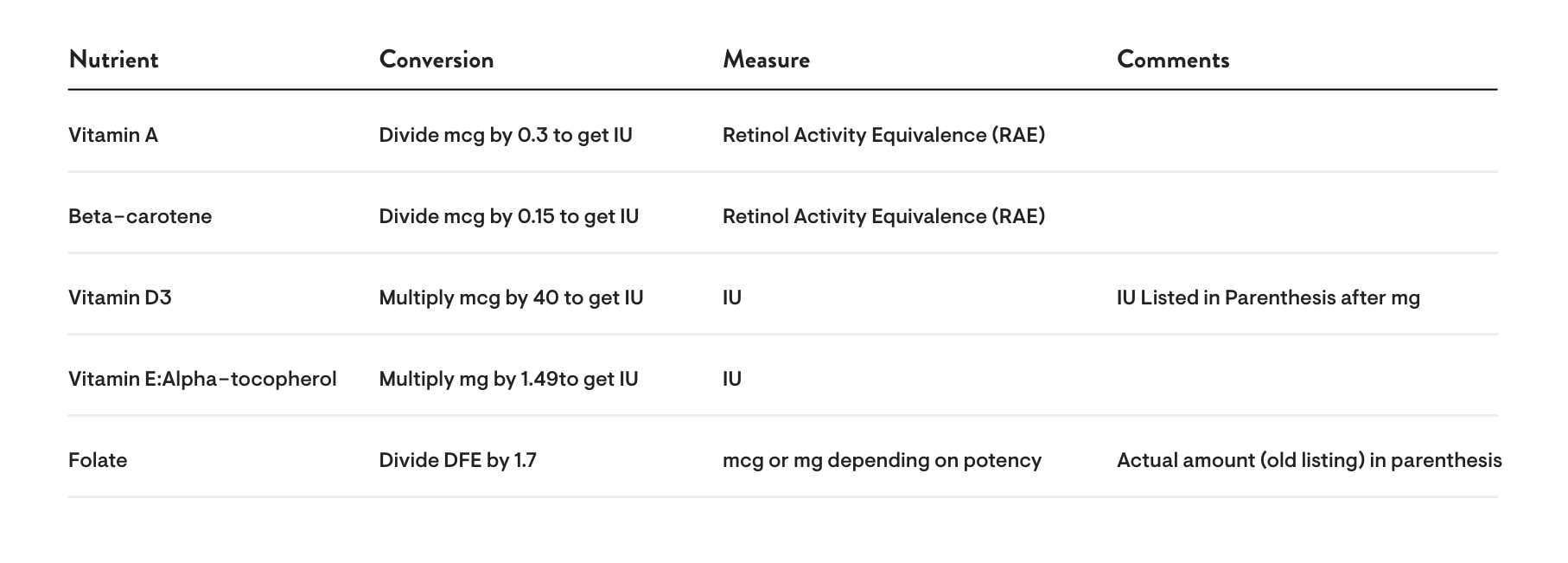
- Vitamin A: Vitamin A is based on the unit of measure known as RAE or Retinol Activity Equivalence. It will appear on the label only as mcg or mg because we are not allowed to list it in IUs, even in parentheses. If you want to know how many IUs are in the product, because that is what you are used to using as a reference point, then converting from mcg to IU is the mcg amount divided by 0.3 = IUs. For example, if there are 750 mcg of vitamin A listed on the label, then you divide 750 by 0.3 and get 2,500 IUs.
- Beta-carotene: Beta-carotene is also based on the RAE unit of measure and will also appear on the label only as mcg or mg. As in the case of vitamin A, we can’t list the amount in IUs, even in parentheses. Converting from IU to RAE for beta-carotene is the mcg amount divided by 0.15 = IUs. For example, if a product has 1,875 mcg of beta-carotene, then you divide 1,875 mcg by 0.15 and get 12,500 IUs.
- Vitamin D3: Vitamin D will also be moving from IUs to mcg on the product label, although for vitamin D we are able to list the IU amount in parentheses. As a point of reference, 1 mcg of vitamin D equals 40 IUs. So Thorne’s D-1,000, which has 1,000 IUs of vitamin D3, converts to mcg by dividing that number by 40: 1,000 IUs divided by 40 equals 25 mcg. This conversion won’t usually be necessary for you to do because we can still list the IUs on the label.
- Vitamin E: Alpha-tocopherol has been listed in IUs on our labels, but will be changing to milligrams (mgs). To convert mgs of alpha-tocopherol to IUs, multiply the listed amount of mgs by 1.49. For example, 268 mg multiplied by 1.49 equals 400 IUs. Alpha-tocopherol is the only tocopherol that can be considered when determining the vitamin E content of a supplement. Where appropriate, the amount of mixed tocopherols in the formula will be listed in parentheses before the vitamin E claim.
- Folate: Folate has a new unit of measure called Dietary Folate Equivalents (DFE). Because supplemental sources of folate are considered more potent than food sources of folate they will have a higher DFE than folate in food sources. So although Thorne has not changed the current formulations, you will see an increased label claim and Percent Daily Value (%DV). To make the conversion you multiply the previous folate claim by 1.7. For example, 1 mg of 5-methyltetrahyrofolate (as listed on a previous label) will now be listed as 1.7 mg DFE, no matter what the folate source (5-MTHF and/or calcium folinate). The actual amount of folate we are putting in the supplements is the same as always and will be noted in parentheses.
- Choline: Choline now has a Percent Daily Value (%DV). Therefore, the amount of choline (not the amount of the entire choline citrate molecule) will be listed. Thus, while it will look like there is less choline in the product, there will actually be the same amount it always had. Because choline citrate is 35 percent choline, whereas an old label might have listed 100 mg of choline citrate, the new label will list “35 mg of choline (as choline citrate).”
The following chart summarizes the conversions for vitamin A, beta-carotene, vitamin D, vitamin E, and folate:
 The following is a comparison between the Supplement Facts Box on a previous label of our popular Basic Nutrients V product – with the above five nutrients highlighted in color – and the Supplement Facts Box for Basic Nutrients V revised according to the new FDA labeling regulations:
The following is a comparison between the Supplement Facts Box on a previous label of our popular Basic Nutrients V product – with the above five nutrients highlighted in color – and the Supplement Facts Box for Basic Nutrients V revised according to the new FDA labeling regulations:

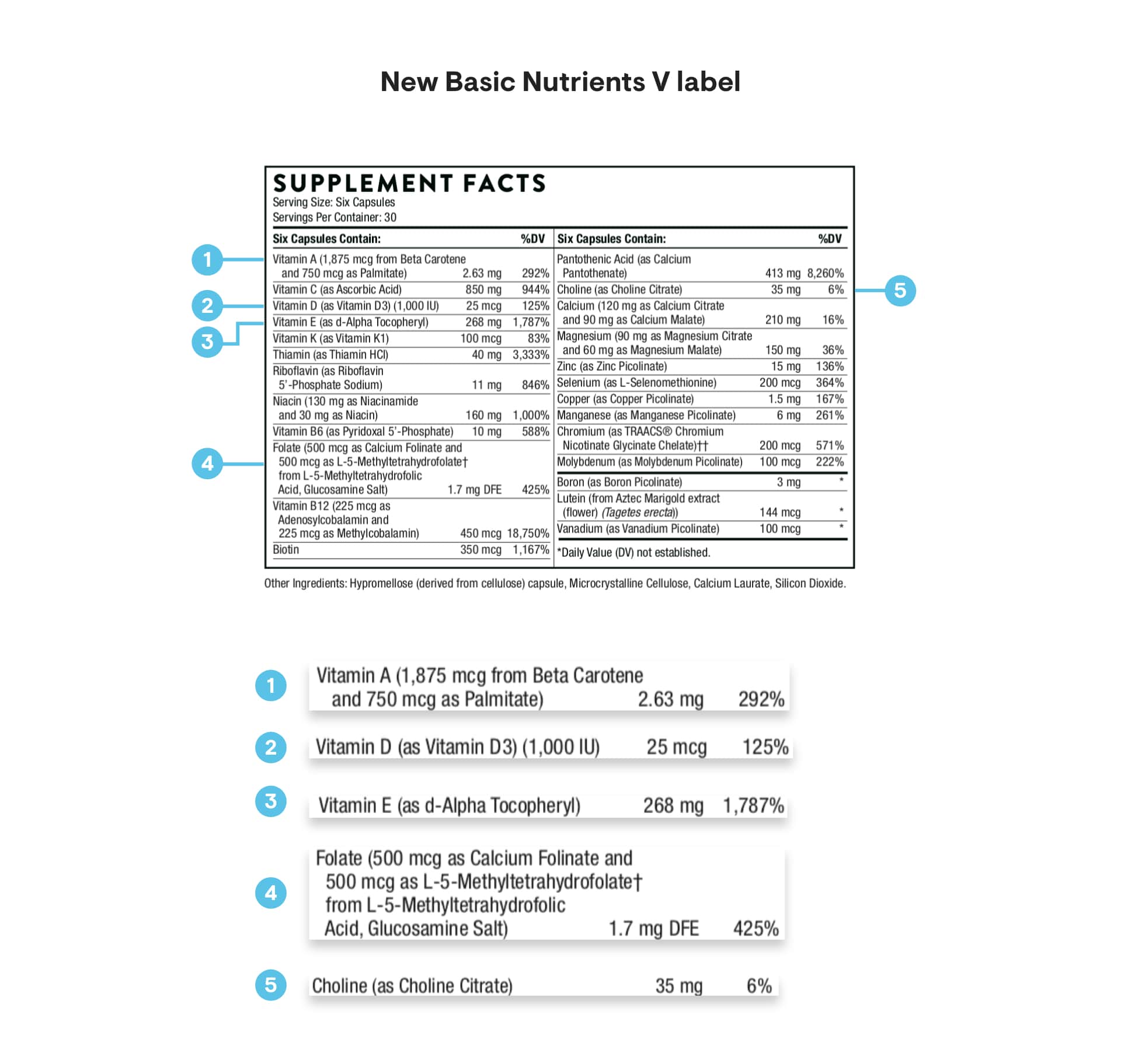
What are some of the other changes you might see?
Other changes in the Supplement Facts Box might not be so obvious, but are still important to keep in mind and help improve ingredient transparency. Certain nutrients that occurred naturally in a product or one of its ingredients will now appear on the label. These four nutrients are:
Iron, Calcium, Potassium and Vitamin D: These represent nutrients that are found naturally in a product, such as a protein powder, but that we don’t actually add to the product as ingredients. If one serving of one of these nutrients in a product contains 2% or more of the FDA’s Reference Daily Intake (RDI; listed on the label as %DV), then it must be listed in the Supplement Facts Box. For example, going forward you now might see a listing for iron in one of our protein powders. If the iron is listed without the source of the iron – for example, simply being listed as iron, and not as iron bisglycinate – then that means the iron is naturally occurring in the protein powder or the flavoring. Our MediClear® suite is an example of this. The Supplement Facts Box on each MediClear product will now list iron as a dietary ingredient because iron occurs naturally in the protein powder and flavoring.
Ingredients not added for therapeutic value: Another example of the new FDA regulations requiring calcium, iron, potassium, and vitamin D to be listed is whether or not the ingredient is being included in the formula for its therapeutic value – calcium in Calcium D-Glucarate, for example. The calcium is not in either product as a calcium source to help build bones; rather, it is present to make the material it is bound to more stable. Nevertheless, if the amount of calcium in one serving is 2% of the %DV or more, it will be listed on its own line in the Supplement Facts Box with the claimed amount.
Added sugars: When a product contains sugar, the number of grams of sugar is always listed. However, under the former FDA labeling regulations, all sugars were considered together. Under the new FDA labeling regulations, “added sugars” will be listed separately from sugars that are naturally occurring in an ingredient.
Other ingredients are no longer required to be listed separately: Some substances that used to appear in the Supplement Facts Box are no longer required so they have been removed from having their own separate line. One example is chloride in Thorne's Joint Support Nutrients – the chloride is part of the glucosamine sulfate-potassium chloride complex. Although chloride is still in the product as part of the potassium chloride that is necessary to stabilize glucosamine sulfate, it is no longer listed as a separate ingredient in the Supplement Facts Box. This is quite helpful because listing chloride on the former product labels made it look like chloride was a therapeutic ingredient.
And starting in 2020 you will need more or less of certain nutrients – so says the feds
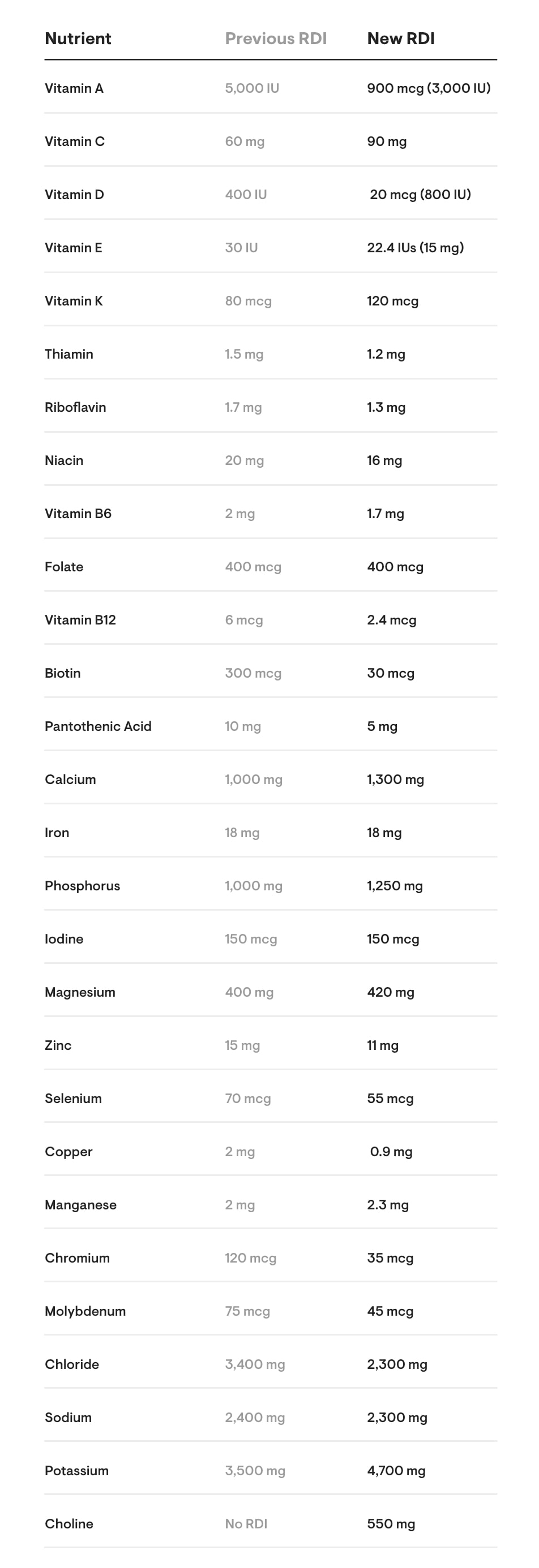
The FDA has established new Percent Daily Values (%DV) for some nutrients. What this means is that the FDA has determined an increase or a decrease in the daily recommendation of a particular nutrient is necessary to maintain health. Therefore, even if the amount of a nutrient in a formula does not change, its %DV might change. The RDIs for nearly all nutrients have been revised by the FDA – some more than others. Below is a chart of the FDA’s new RDIs compared with the former ones.
Comparison chart of former RDIs versus new RDIs for adults and children 4+ years:
We trust that the above information will make it easier for you to interpret the Supplement Facts Box throughout our entire product line as the new FDA labeling regulations begin to be implemented in the market place.

