Essential Amino Acids vs. Branched-Chain Amino Acids

What exactly are branched-chain amino acids (BCAAs)? Should you be supplementing with them? Do BCAAs work? Researchers continue to question the effectiveness of taking a supplement that just contains BCAAs versus taking a supplement that contains all of the essential amino acids.
Although BCAAs are an extensively-researched option for supporting muscle health and recovery, this blog aims to shed some light on this debate and minimize confusion.
Let’s start with the basics: what are amino acids?
The protein we eat in our food – from meat, dairy (including whey), legumes, etc. – is broken down into 20 different amino acids in our digestive tract. Amino acids are often referred to as the building blocks of life because it’s amino acids that make up our DNA, build muscles, and provide structure and therefore, function, to our tissues and organs.
Our bodies use these 20 amino acids to reassemble proteins in thousands of combinations, much like we use the 26 letters of the alphabet to form an endless number of words.
Although amino acids provide the physical structure of our bodies, they also provide energy, support digestion, and are involved in numerous enzymatic reactions, hormonal signals, and neurotransmitter messaging.
Although protein and amino acids are generally discussed for their ability to initiate muscle building and muscle repair, only about 10% of the protein we digest is available for this function.
On the other hand, 50% of the protein we eat is directed toward the liver or GI tract, and 40% is used for energy production, the formation of neurotransmitters, and supporting our waste management system.
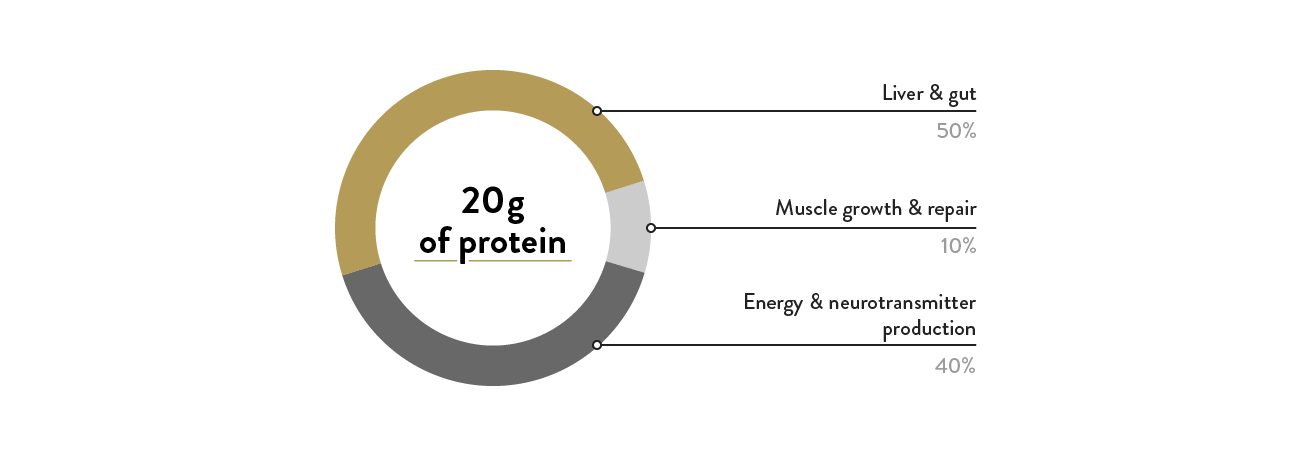
Should I be supplementing with BCAAs?
While our bodies can produce 11 amino acids using other amino acids to do so (non-essential amino acids), nine of the 20 amino acids are considered essential amino acids (EAAs) because these amino acids must come from diet or supplementation since the body can’t make them. Of these nine, three are referred to as BCAAs because of their chemical structure.
Athletes consume these three BCAAs because of their unique structure, which allows them to skip the normal digestion processes and become quickly available in the muscle to be used as energy or to be saved in the amino acid pool for later metabolism.
In times of illness, injury, growth, or stress – including those caused by sports or competition – our need for six of the 11 non-essential amino acids exceeds the amount we can produce; we call this group conditionally essential amino acids.
The answer to whether or not you should be supplementing amino acids depends on how much high-quality protein you consume and when, and how much exercise, stress, or injury/illness you experience.
Each amino acid is separately (and in combination) linked to various functions in our body. For example, glutamine is one of the few amino acids that can cross the blood-brain barrier while also supporting a healthy intestinal lining and immune function.* Tryptophan is needed to create serotonin, which is why it is associated with restful sleep.*
- Essential Amino Acids: Histidine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan, Isoleucine†, Leucine†, and Valine†
- Nonessential Amino Acids: Alanine, Asparagine, Aspartic Acid, Glutamic Acid
- Conditionally Essential Amino Acids: Arginine (essential in children, not in adults), Cysteine, Glutamine, Glycine, Proline, Serine, and Tyrosine
†Branched-Chain Amino Acids
Do they work?
There has been a buzz about BCAAs, especially leucine, in the athletic community since research determined that leucine is responsible for initiating the muscle recovery process after training.
Exercise at a high enough intensity or a long enough duration can break down muscles, which continues until the body is given a signal to start the recovery process.
Leucine provides this signal to switch from a catabolic state to an anabolic state – where the body stops breaking down muscle and starts to rebuild it. The goal is to receive this signal as soon as possible after training is completed; i.e., the sooner you switch from breaking down to building up, the better you optimally repair and recover.
More recently, researchers have sought to determine if leucine is the only key amino acid needed for muscle repair and recovery, or whether all the BCAAs or even all of the EAAs should be present in the bloodstream for optimal repair and recovery.
A 2016 study by Moberg et al compared the impact of water (placebo), leucine, BCAAs (which includes leucine), and all EAAs (which include BCAAs) in resistance-trained men completing a training protocol.
The results found that BCAAs provided alone or in the full mix of EAAs stimulated repair and recovery better than water or leucine alone. Building on this information, other researchers completed a study on the magnitude of the difference between the groups.
They found that BCAAs stimulated muscle protein synthesis 22% more than the water group.
While this improvement is significant, it is only 50% of the muscle protein synthesis improvement obtained when all of the essential amino acids were provided. The researchers concluded that, while BCAAs are essential to start the recovery process, they do not offer the full complement of building blocks needed to promote new muscle growth and repair – all EAAs need to be present for optimal results.
Putting it all together
Many factors go into choosing a post-workout protein that contains a full complement of essential amino acids. Although most athletes choose a whey protein isolate to support muscle repair and recovery,* researchers have shown that when essential amino acids that contain about five grams of leucine are added to a low protein beverage, the ability to stimulate protein synthesis compared favorably to whey protein isolate.
Therefore, athletes in weight class sports, or individuals requiring strict management of calories, or individuals with problems tolerating whey protein should look to a complete EAA product to support their training.
References
- Moberg M, Apró W, Ekblom B, et al. Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise. Am J Physiol Cell Physiol 2016;310(11):C874-C884.
- Jackman S, Witard O, Philp A, et al. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Front Physiol 2017;8:390.
- Murphy C, Saddler N, Devries M, et al. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am J Clin Nutr 2016;104(6):1594-1606.
- Churchward-Venne T, Burd N, Mitchell C, et al. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol 2012;590(11):2751-2765.
- Stokes T, Hector A, Morton R, et al. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients 2018;10(2). pii: E180. doi: 10.3390/nu10020180.
- Witard O, Jackman S, Breen L, et al. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 2014;99(1):86-95.

